Factors Governing the Stability of Emulsions
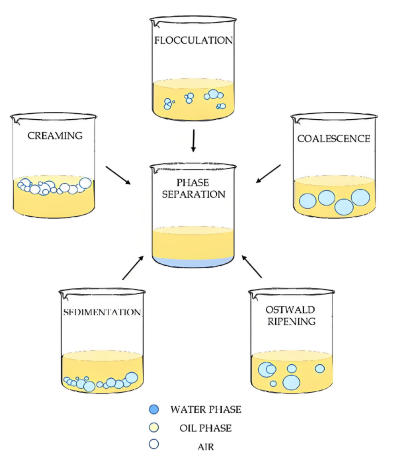
In practical applications, the stability of an emulsion refers to the dispersed phase droplets’ ability to resist coalescence. Among the metrics for gauging emulsion stability, the rate of coalescence among dispersed droplets is paramount; it can be determined by measuring how the number of droplets per unit volume changes over time. As droplets in the emulsion merge into larger ones and ultimately lead to breaking, the speed of this process hinges chiefly on the following factors: the physical properties of the interfacial film, electrostatic repulsion between droplets, steric hindrance from polymer films, viscosity of the continuous phase, droplet size and distribution, phase volume ratio, temperature, and so forth.
Of these, the physical nature of the interfacial film, electrical interactions, and steric hindrance are the most critical.
(1) Physical Properties of the Interfacial Film
Collision among dispersed-phase droplets is the prerequisite for coalescence. Coalescence proceeds unceasingly, shrinking small droplets into larger ones until the emulsion breaks. In the course of collision and merging, the mechanical strength of the droplet’s interfacial film stands as the foremost determinant of emulsion stability. To endow the interfacial film with substantial mechanical strength, it must be a coherent film—its constituent surfactant molecules bound together by strong lateral forces. The film must also possess good elasticity, so that when localized damage occurs from droplet collisions, it can spontaneously mend itself.
(2) Electrical Interactions
Droplet surfaces in emulsions may acquire certain charges for various reasons: ionization of ionic surfactants, adsorption of specific ions onto the droplet surface, friction between droplets and the surrounding medium, etc. In oil‑in‑water (O/W) emulsions, the charging of droplets plays a vital role in forestalling aggregation, coalescence, and eventual breaking. According to colloid stability theory, van der Waals forces draw droplets together; yet when droplets approach closely enough for their surface double layers to overlap, electrostatic repulsion impedes further closeness. Clearly, if repulsion outweighs attraction, droplets are less prone to collide and coalesce, and the emulsion remains stable; otherwise, coalescence and breaking ensue.
As for water‑in‑oil (W/O) emulsions, water droplets carry little charge, and because the continuous phase has a low dielectric constant and a thick double layer, electrostatic effects exert only a minor influence on stability.
(3) Steric Stabilization
When polymers serve as emulsifiers, the interfacial layer becomes substantially thicker, forming a robust lyophilic shield around each droplet—a spatial barrier that hinders droplets from drawing near and making contact. The lyophilic nature of polymer molecules also entraps a considerable amount of continuous‑phase liquid within the protective layer, rendering it gel‑like. Consequently, the interfacial region exhibits heightened interfacial viscosity and favorable viscoelasticity, which help prevent droplet merging and preserve stability. Even if some coalescence occurs, polymer emulsifiers often assemble at the diminished interface in fibrous or crystalline forms, thickening the interfacial film and thereby staving off further coalescence.
(4) Uniformity of Droplet Size Distribution
When a given volume of dispersed phase is broken into droplets of varying sizes, the system comprising larger droplets has a smaller total interfacial area and thus lower interfacial energy, conferring greater thermodynamic stability. In an emulsion where droplets of both large and small sizes coexist, small droplets tend to shrink while large ones grow. If this progression continues unchecked, breaking will eventually occur. Hence, an emulsion with a narrow, uniform droplet size distribution is more stable than one whose average droplet size is the same but whose size range is broad.
(5) Influence of Temperature
Temperature variations can alter interfacial tension, the properties and viscosity of the interfacial film, the relative solubility of the emulsifier in the two phases, vapor pressure of the liquid phases, and the thermal motion of dispersed droplets. All these changes can affect emulsion stability and may even induce phase inversion or breaking.
Post time: Nov-27-2025